What is lipoprotein(a), or Lp(a)?
Lp(a) is a distinct lipoprotein and is the only lipoprotein particle that contains apolipoprotein(a).1 It is made by the liver, and consists of fat and proteins which carry cholesterol and other lipids through the bloodstream.2 Elevated Lp(a) is an independent and causal risk factor for atherosclerotic cardiovascular disease (ASCVD).1,3

It is estimated that elevated Lp(a) levels of ≥125 nmol/L or 50 mg/dL1 is associated with 5% of CVD events,4 increasing likelihood of myocardial infarction (MI) by 3–4x,5 ischaemic stroke (IS) by 1.6x6 and aortic valve stenosis (AVS) by 3x,6 compared to those with low Lp(a) levels. Elevated Lp(a) levels can also occur in chronic kidney disease and liver disease.7

Elevated Lp(a) levels are associated with cardiovascular disease risk in all ethnic groups.8 However, individuals of Black origin have been found to have the highest Lp(a) levels of all ethnicities, followed by South Asian, White, Hispanic and East Asian populations.8
Lp(a) levels

Levels are predominately (70-90%) genetically determined, with an autosomal co-dominant inheritance pattern.1,4,9,10

Non-genetic factors can influence levels, including menopause due to declining oestrogen levels.2
Kidney disease is associated with both an increased risk of vascular disease and an acquired elevation in Lp(a) levels.7
Lp(a) levels remain relatively consistent over a lifetime.4,10 One Lp(a) measurement is sufficient in most patients unless a secondary cause of elevated Lp(a) is suspected such as untreated overt hypothyroidism, chronic kidney disease, end stage renal failure on dialysis, nephrotic syndrome, autoimmune disorders and treatment with growth hormone. Twofold increases in Lp(a) levels can also be seen in pregnancy.10,27,28 It can also increase post-menopausally.31
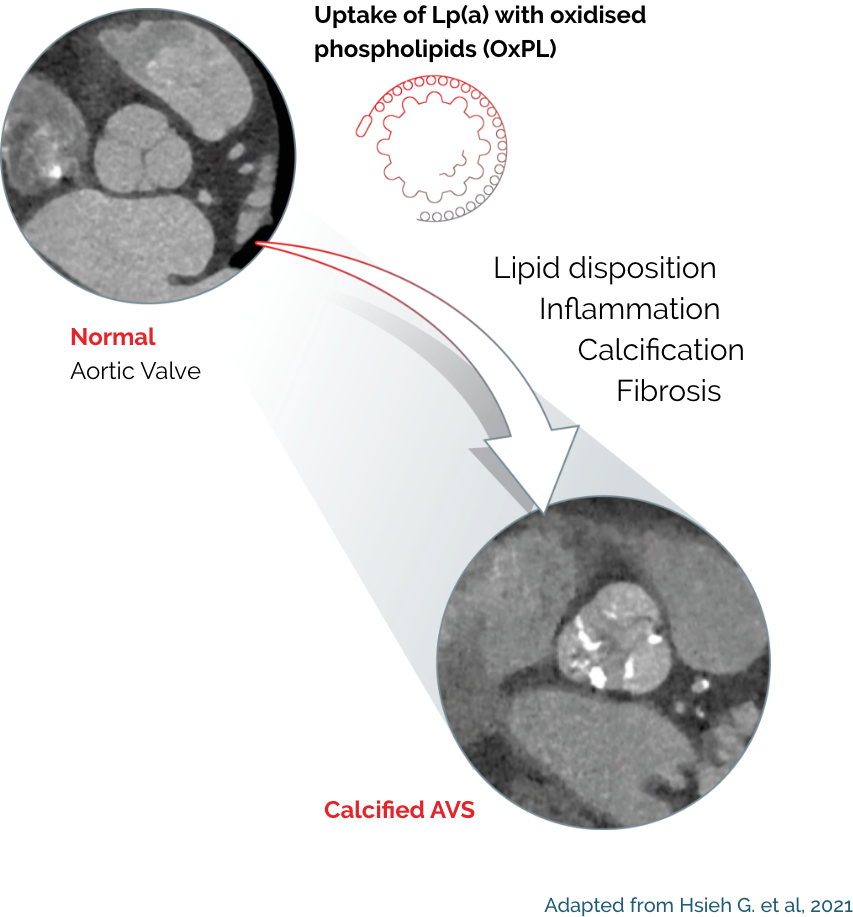
Lp(a) is a risk factor for calcific aortic valve stenosis as well as ASCVD
Elevated Lp(a) is a risk factor for calcific aortic valve stenosis (CAVS), the most common form of valvular heart disease in developed countries. Pathophysiologic, epidemiological, and genetic studies over the past two decades have provided strong evidence that Lp(a) is an important mediator of calcific aortic valvular disease;11 individuals with elevated Lp(a) are at 3x risk of developing AVS compared to those with low Lp(a) levels.12
Lp(a)-targeted therapies are needed to address Lp(a)-associated CV disease risk
Currently there are no licensed therapies for targeting Lp(a) levels. Observational and Mendelian randomisation studies suggest that an absolute Lp(a) reduction of 50-100 mg/dL (approximately 105 –214 nmol/L) is needed to effectively lower Lp(a)-associated CV disease risk.13,14
Therapies such as niacin, proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors and mipomersen show modest reduction of Lp(a) levels by 20–30%. Notably these are not licensed for use in targeting elevated Lp(a). Similarly, apheresis leads to a time-average reduction of 30% to 35%. Apheresis is available in the UK but is not the standard of care.15
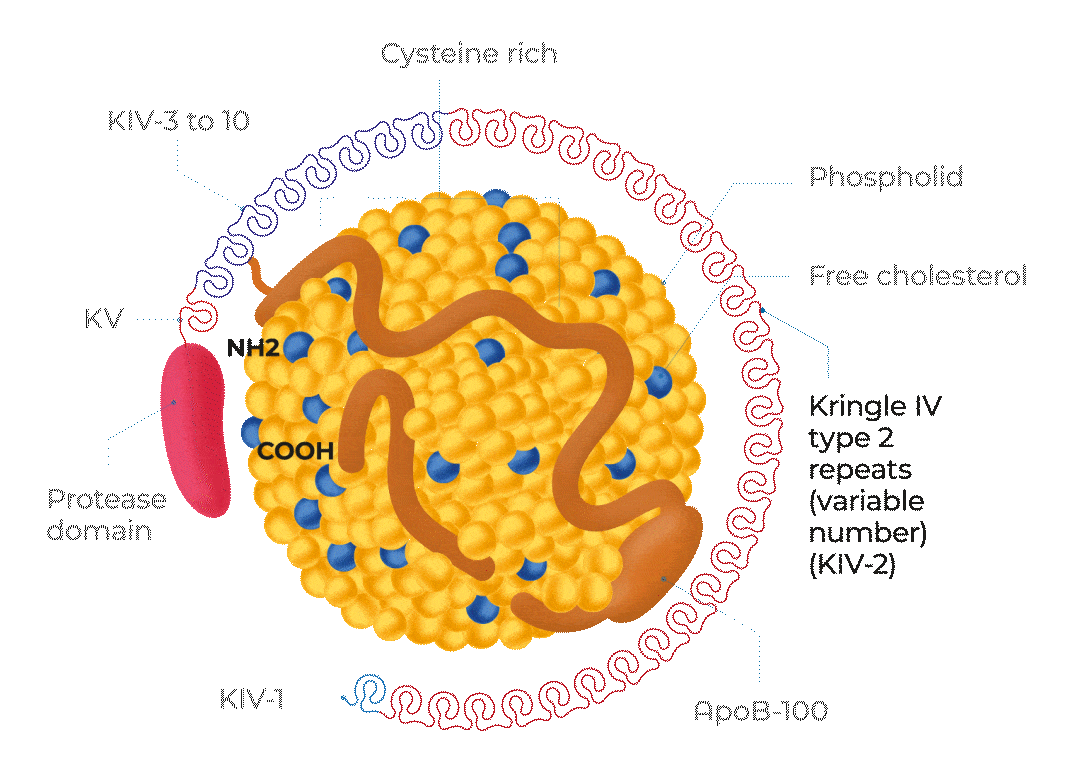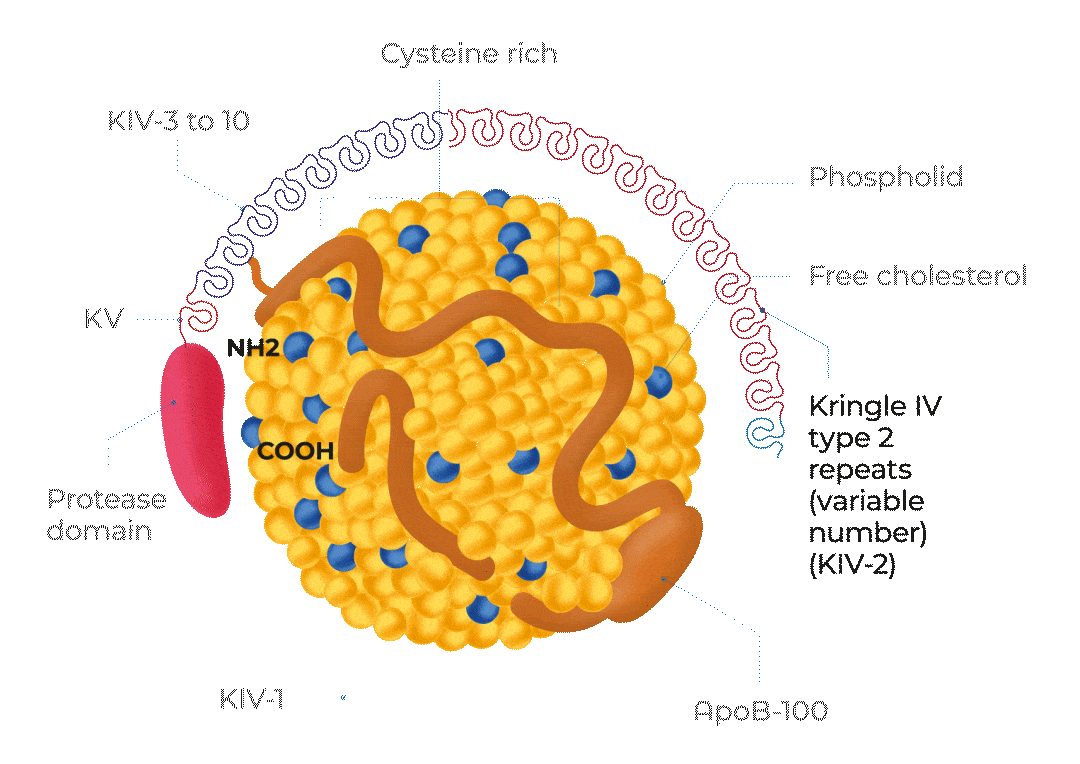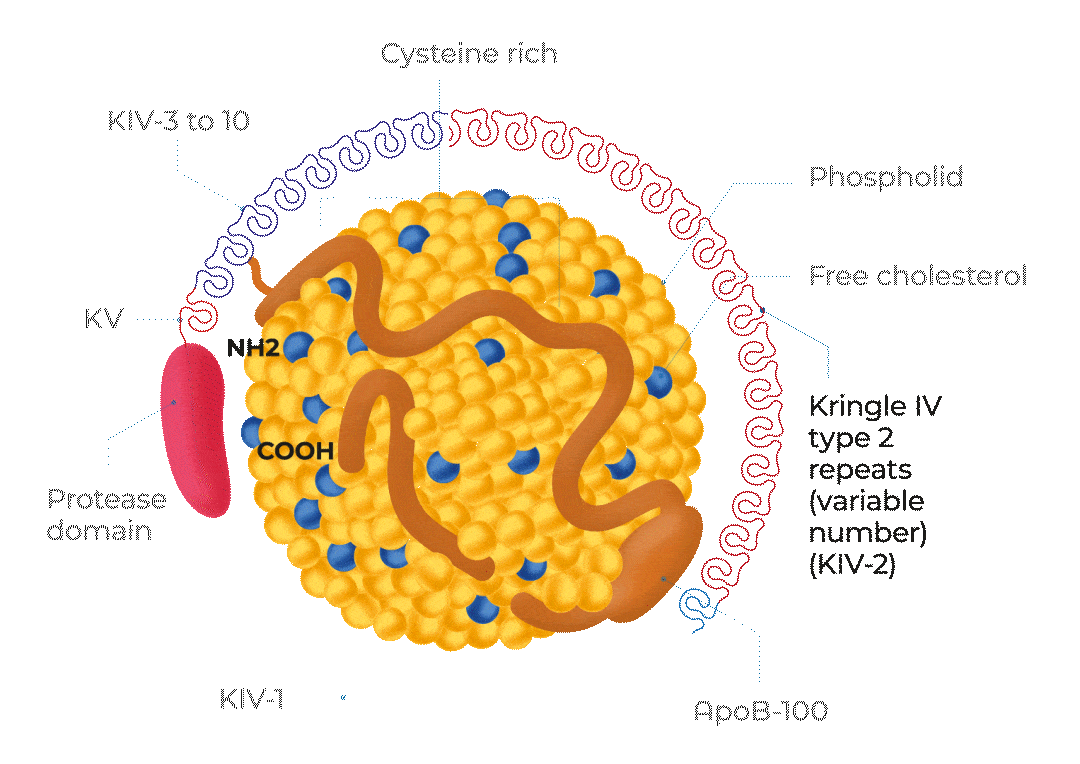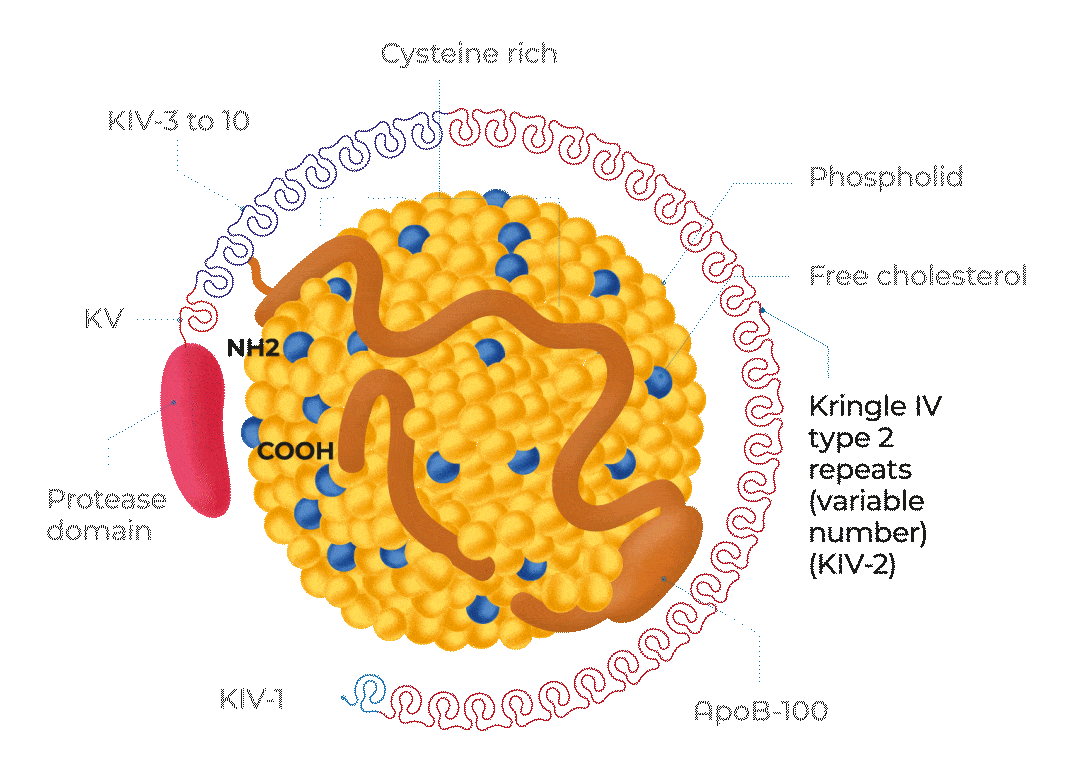
KV, Kringle 5; KIV-1, Kringle 4 type 1; KIV-3 to 10, Kringle 4 types 3 to 10;
ApoB-100, Apolipoprotein B100; NH2, NH2-terminal; COOH, C-terminus
Adapted from Cegla J et al, 2019
The structure of Lp(a)
Lp(a) is composed of one molecule of a LDL-particle containing apoB-100 and one molecule of a large highly polymorphic glycoprotein named apo(a). This also includes cholesterol, cholesteryl esters, phospholipids and triglycerides.29
A characteristic feature of apo(a) is the presence of loop-like structures called kringles. Kringle domains are triple loop structures stabilised by three internal disulfide bonds and are also present in other coagulation factors, such as plasminogen (PLG), prothrombin, urokinase, and tissue-type PLG activators. In contrast to PLG, the linker domain between kringles is glycosylated in apo(a). The apo(a) is synthesised by the liver. The two components of Lp(a) are covalently linked together by a disulfide bond between apoB-100 of the LDL moiety and one of the kringle domains in apo(a). The assembly of Lp(a) is believed to occur at the hepatocyte cell membrane surface.30
Lp(a) levels are controlled by the LPA gene locus. The molecular mass of apo(a) can vary between 275 and 800 kDa due to the inheritance of >40 different allelic variants of the LPA gene encoding different numbers of kringle IV type 2 repeat sequences in this polypeptide.1
The rate of apo(a) synthesis and secretion is inversely related to its molecular mass, and consequently, individuals who produce the lower molecular mass apo(a) isoforms have higher serum Lp(a) levels than those who produce the higher molecular mass isoforms.1
Elevated Lp(a) may drive fast-progressing and vulnerable atherosclerotic plaques
In patients with advanced stable coronary artery disease (CAD), elevated Lp(a) was associated with accelerated progression of the necrotic core.16
Patients with elevated Lp(a) may exhibit a more severe disease presentation, which refers to the complex atherosclerotic lesions that are more difficult to treat.17
In patients with acute coronary syndrome (ACS), elevated Lp(a) drives a higher prevalence of vulnerable plaque characteristics, including thin-cap fibroatheroma.18
Elevated Lp(a) levels are independently associated with plaque progression and vulnerable plaque characteristics.19,20
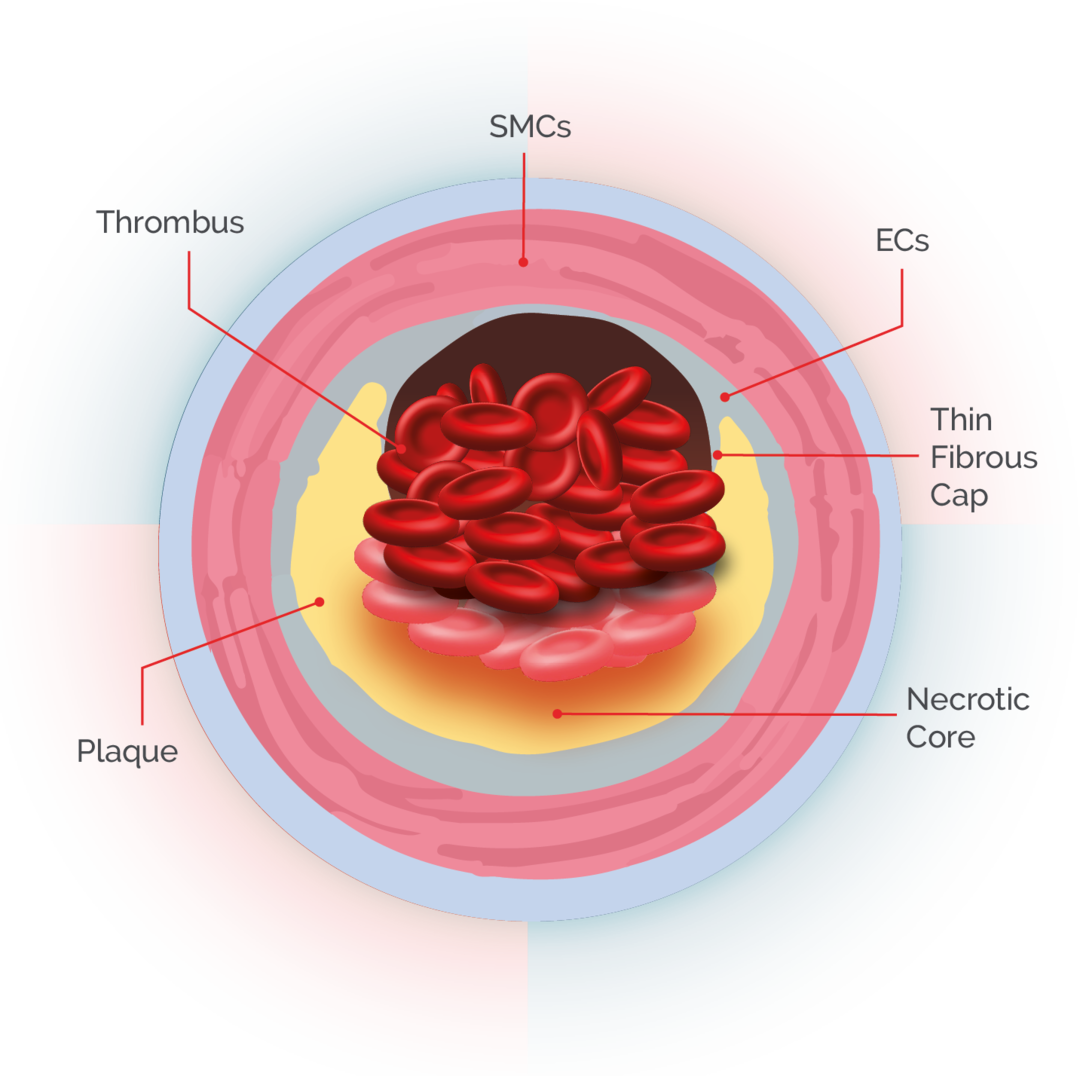
ACS, acute coronary syndrome; CAD, coronary artery disease; ECs, endothelial cells;
FH, familial hypercholesterolemia; Lp(a), lipoprotein(a); SMCs, smooth muscle cells.
How does elevated Lp(a) affect cardiovascular health (CV)?
- Elevated Lp(a) is independently and causally associated with increased risk of myocardial infarction (MI), peripheral arterial disease (PAD), ischemic stroke (IS), aortic valve stenosis (AVS), and cardiovascular (CV) mortality.10,17,21
- In patients undergoing coronary angiography, elevated Lp(a) levels are associated with a greater rate of significant obstructive disease and three-vessel disease.22
- In patients with ASCVD and elevated Lp(a), circulating inflammatory and vascular dysfunction markers were elevated; Lp(a) amplifies atherothrombotic risk by driving monocyte-mediated tissue factor expression and activation.23
Elevated Lp(a) is independently and causally associated with increased risk of MI, PAD, IS, and CV mortality
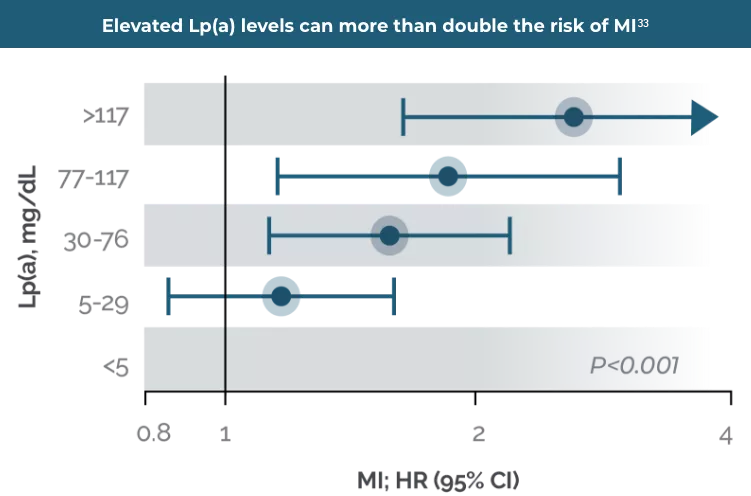
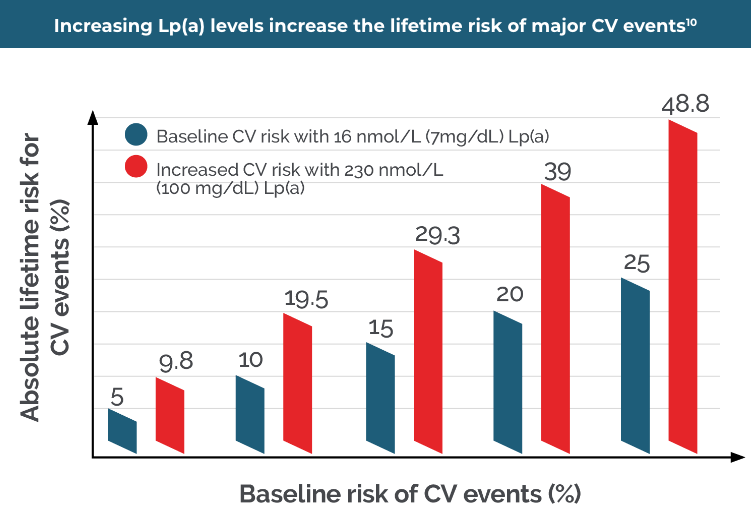
By NOT measuring an Lp(a) level, the lifetime risk of a CV event could be substantially underestimated34
Lp(a) increases cardiovascular risk through three main mechanisms24
Pro-atherogenic
Oxidised phospholipids (OxPLs) on Lp(a) likely contribute to its pro-inflammatory and pro-atherogenic potential, and pro-osteogenic potential in coronary artery vasospasm.25,26
- Endothelial cell binding
- Upregulation of adhesion molecules
- Smooth muscle cell proliferation and proteoglycan matrix binding
- Foam cell formation
- Necrotic core formation
- Lesion calcification

Pro-inflammatory
Lp(a) elicits pro-inflammatory responses of vascular and immune cells, which are key initiating steps in atherosclerosis.25,26
- Macrophage Interleukin 8 (IL-8) expression
- Monocyte cytokine release
- Oxidised phospholipids
- Monocyte chemotaxis/ transmigration
- Lp(a) carries Monocyte Chemoattractant Protein-1 (MCP-1)
Prothrombotic
A pro-thrombotic role for Lp(a) has been hypothesised since the discovery of apo(a) sharing extensive homology (75-99%) with the blood clotting protein plasminogen. Potential pro-thrombotic mechanisms of Lp(a) include promotion of platelet aggregation, accelerated coagulation, more resistant fibrin structures, impaired fibrinolysis and vulnerable plaques.25,26,32
- Plasminogen activation
- Fibrin degradation
- Endothelial cell plasminogen activator inhibitor-1 (PAI-1) expression
- Tissue factor pathway inhibitor (TFPI) activity
- Platelet activation
A 2024 study revealed that elevated Lp(a) was associated with a more severe presentation of coronary artery disease (CAD).
Patients with CAD suffer with progressive plaque build-up in the walls of coronary blood vessels, which restricts blood flow and may result in serious cardiovascular outcomes such as angina and MI. In the study, it was observed that elevated Lp(a) was associated with a higher proportion of patients with prior CAD, prior interventions on coronary blood vessels, and more diseased blood vessels. These collectively form what is considered a ‘severe’ clinical presentation of CAD, meaning a greater likelihood of adverse clinical outcomes. Awareness of Lp(a) levels in patients with CAD may have implications in their clinical management.17
Lp(a) is the strongest genetic driver for CAD identified so far.30
References:
- Cegla J, et al. Atherosclerosis. 2019. 291:62-70
- HEART UK. What is Lp(a)? Available from: https://www.heartuk.org.uk/genetic-conditions/high-lipoproteina [Last accessed July 2025]
- Reyes-Soffer et al. Arteriosclerosis, Thrombosis, and Vascular Biology. 2022;42:e48–e60
- Lp(a) Taskforce. A call to action from the Lipoprotein(a) Taskforce. Available from: https://www.heartuk.org.uk/downloads/health-professionals/a-call-to-action-from-the-lipoprotein(a)-taskforce---august-2023.pdf [Accessed July 2025]
- Kamstrup et al. J Am Coll Cardiol 2013;61:1146–1156
- Wilson et al. J Clin Lipidol. 2019;13(3):374–392
- Hopewell JC, et al. J Lipid Res. 2018 Apr;59(4):577-585.
- Mehta A, et al. Atherosclerosis. 2022 May:349:42-52.
- Kenet G, et al. Circulation. 2010 Apr 27;121(16):1838-47.
- Kronenberg F, et al. Eur Heart J. 2022 Oct 14;43(39):3925-3946.
- Hsieh G, et al. Curr Opin Cardiol. 2021 Sep 1; 36(5): 542-548.
- Langsted et al. J Am Coll Cardiol. 2019;74(1):54–66; 4
- Burgess et al. JAMA Cardiol. 2018;3(7):619–627;
- Madsen et al. Arterioscler Thromb Vasc Biol. 2020;40(1):255–266.
- Tsimikas S, et al. J Am Coll Cardiol. 2017;69(6): 692-711.
- Kaiser Y, et al. J Am Coll Cardiol. 2022;79(3):223–233.
- Leistner D & Laguna-Fernandez A, et al. Eur J Prevent Cardiol. 2024;(In Press)
- Kato A, et al. Int J Cardiol Heart Vasc. 2022;43:101120.
- Verweij SL, et al. J Clin Lipidol. 2018;12(3):597–603.e1
- Garg PK, et al. J Cardiovasc Comput Tomogr. 2021;15(2):154–160.
- Arsenault BJ & Kamstrup PR. Atherosclerosis. 2022;49:7–16
- Nicholls SJ, et al. J Lipid Res. 2010;51(10):3055–3061.
- Robert S. Rosenson, et al. Lipoprotein (a) integrates monocyte-mediated thrombosis and inflammation in atherosclerotic cardiovascular disease, Journal of Lipid Research, Volume 66, Issue 6, 2025, 100820, ISSN 0022-2275, https://doi.org/10.1016/j.jlr.2025.100820.
- Reyes-Soffer et al. Am J Prev Cardiol. 2024;18:199651.
- van der Valk FM, et al. Circulation. 2016;134(8):611–624
- Schnitzler JG, et al. CircRes. 2020;126(10):1346–1359;
- Missala I, Kassner U, Steinhagen-Thiessen E. A systematic literature review of the association of lipoprotein(a) and autoimmune diseases and atherosclerosis. Int J Rheumatol 2012; 2012: 480784.
- Patel AP, Wang M, Pirruccello JP, et al. Lp(a) (Lipoprotein[a]) concentrations and incident atherosclerotic cardiovascular disease: new insights from a large National Biobank. Arterioscler Thromb Vasc Biol 2021; 41: 465–474.
- Schmidt K, Noureen A, Kronenberg F, Utermann G. Structure, function, and genetics of lipoprotein (a). J Lipid Res. 2016 Aug;57(8):1339-59. doi: 10.1194/jlr.R067314. Epub 2016 Apr 13. PMID: 27074913; PMCID: PMC4959873.
- Clarke R, et al. N Engl J Med. 2009;361(26):2518‒2528.
- Corral P, Matta MG, Aguilar-Salinas C, Mehta R, Berg G, Ruscica M, Schreier L. Lipoprotein(a) throughout life in women. Am J Prev Cardiol. 2024 Oct 29;20:100885. doi: 10.1016/j.ajpc.2024.100885. PMID: 39624479; PMCID: PMC11609252.
- Boffa, M. B., & Koschinsky, M. L. (2016). Lipoprotein (a): Truly a direct prothrombotic factor in cardiovascular disease? Journal of Lipid Research, 57(5), 745–757. https://doi.org/10.1194/jlr.R060582
- Kamstrup PR, et al. JAMA. 2009;301(22):2331‒2339.
- Kronenberg F, et al. Atherosclerosis. 2023;374:107–120.
UK | July 2025 | FA-11414800

