Lipoprotein(a) levels
Measurement of Lp(a)
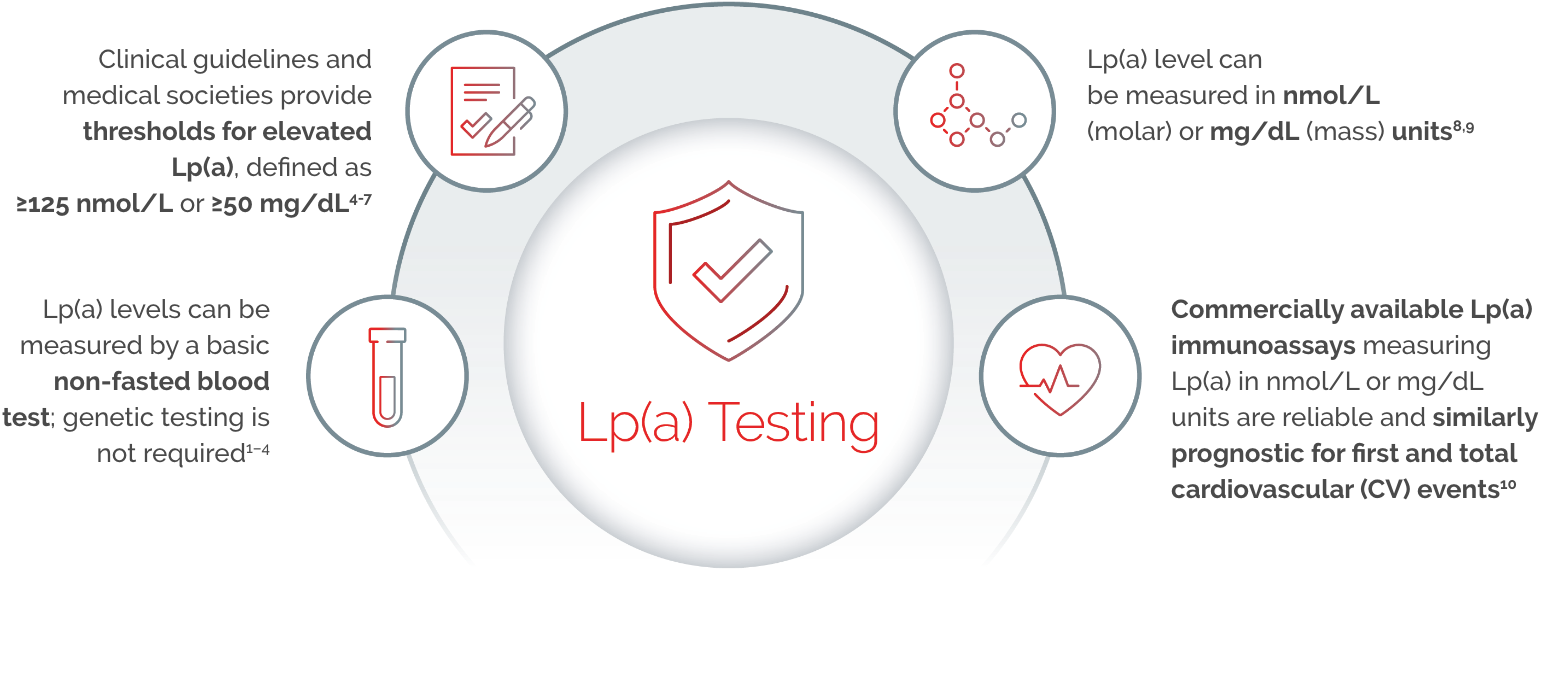
Lp(a) expressed in mass units (mg/dL) is indicative of the mass of the entire particle, whereas nmol/L reflects the particle number. Because of the heterogeneity in apolipoprotein(a) [apo(a)] size and the presence in most individuals of two different, genetically determined apo(a) isoform sizes, standardisation using a single calibrant material is impossible.11
Serum Lp(a) levels should be measured using a method where the effect of isoform size has been minimised using appropriate antibodies with calibrators certified for traceability of Lp(a) values to the World Health Organization / The International Federation of Clinical Chemistry (WHO/IFCC) reference material.
HEART UK and The Association for
Laboratory Medicine consensus guidance11,12
HEART UK and The Association for Laboratory Medicine published guidance on standardising lipid testing and reporting, including for Lp(a).
- As per guidance from HEART UK, Lp(a) measurement should be considered in patients with
(a) A personal or family history of premature atherosclerotic cardiovascular disease.
(b) First degree relatives with raised serum Lp(a).
(c) Familial hypercholesterolaemia (FH), or other genetic dyslipidaemias.
(d) Calcific aortic valve stenosis.
(e) Moderate (10–15%) 10-year risk of cardiovascular event. - A single measurement of Lp(a) is adequate in most patients unless a secondary cause for elevated Lp(a) is identified.
- Denka based assays with calibrators traceable in nmol/L to WHO/IFCC reference material are the only recommended assays at present.
- Results should be reported in nmol/L and conversion from mass to molar unit should be avoided.
In a national questionnaire-based survey, based on the responses of 53 UK lipid clinics:13






National effort is required to provide universal access to Lp(a) measurement and to harmonise the clinical application of this data.
How often should you
test patients?
To improve the accuracy of CV risk assessment, a single measurement of serum Lp(a) is sufficient for most patients, unless a secondary cause is suspected.11

References:
- Reyes-Soffer G, et al. Arterioscler Thromb Vasc Biol. 2022;42(1):e48–e60.
- Kronenberg F. Clin Res Cardiol Suppl. 2019;14(Suppl 1):5–12.
- Nordestgaard BG. J Am Coll Cardiol. 2017;70(13):1637–1646.
- Wilson DP, et al. J Clin Lipidol. 2019;13(3):374–392.
- Pearson CG, et al. Can. J. Cardiol. 2021; 37:1129–1150.
- Handelsman Y, et al. Endocr Pract. 2020;26(10):1196–1224.
- Grundy SM, et al. Circulation. 2019;139(25):e1082–e1143.
- Kronenberg F. Atherosclerosis. 2022;349:123–135.
- Kamstrup PR. Clin Chem. 2021;67(1):154–166.
- Szarek M, et al. Circulation. 2023;10.1161.
- Cegla J, et al. Atherosclerosis. 2019. 291:62-70.
- Kenkre JS, et al. Annals of Clinical Biochemistry. 2025;0(0).
- Ansari S, et al. J Clin Lipidol. 2024 Feb 15:S1933-2874(24)00023-0.
UK | July 2025 | FA-11414807

